Atomoxetinum (Atomoxetinum) - Strattera
26 Mar 2018
A drug intended for the treatment of attention deficit hyperactivity disorder (ADD, ADHD). By the mechanism of action, atomoxetine is an inhibitor of norepinephrine reuptake (indirect sympathomimetic of the central action).
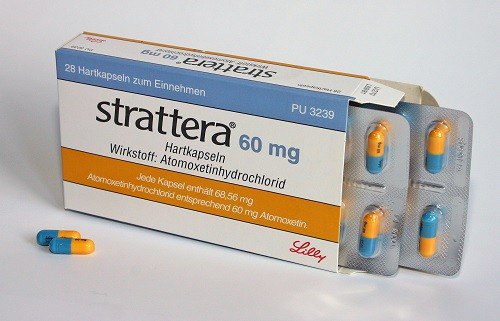
Atomoxetine is approved by the US Food and Drug Administration for the treatment of attention deficit / hyperactivity disorder in children, adolescents and adults. Its effectiveness has not been investigated in children under 6 years. Unlike psychostimulants traditionally prescribed for ADHD, atomoxetine is not a narcotic and does not have the inherent potential for abuse. In clinical trials, it has been shown that atomoxetine is able to provide a stable 24-hour monitoring of ADHD symptoms in both adults and children.
Atomoxetine is also used to treat treatment-resistant depression as an independent remedy or as part of a comprehensive treatment with SSRIs or other drugs.
The therapeutic effects of atomoxetine develop gradually over at least one week. The duration of the course use of the drug should be 6-8 weeks before the decision on its level of effectiveness. Many patients with ADHD, whose disease can not be corrected by psychostimulants, respond to treatment with atomoxetine. Atomoxetine is also more preferred in patients with a variety of mental disorders, those who do not tolerate psychostimulants well, as well as patients with substance abuse in the anamnesis. Psychostimulants based on amphetamine (mixed salts of amphetamine and dextroamphetamine), pure dextroamphetamine, lisdexamphetamine, etc.) are not recommended for use in patients suffering from nervous disorders (such as facial tics, spasms, etc.). In such cases, atomoxetine is the best choice.
Atomoxetine potently and highly selectively inhibits the proteins of the presynaptic membrane carrying out the re-uptake of norepinephrine, serotonin and dopamine. The binding constants (Ki) are 5, 77 and 1451 nM, respectively. In studies of tissue microdialysis, it has been shown that atomoxetine triply increases levels of norepinephrine and dopamine in the prefrontal cortex, but does not alter the level of dopamine in the striatum and adjacent nucleus. Also, atomoxetine in therapeutic doses acts as an NMDA receptor antagonist. The role of such antagonism in the therapeutic profile of atomoxetine has yet to be clarified, however, fresh sources of literature imply that the dysfunction of the glutamatergic system may play a role in the etiology and pathogenesis of ADHD.
Atomoxetine does not have a clinically significant affinity for serotonin, acetylcholine and adrenergic receptors.
Side effects include dry mouth, fatigue, irritability, nausea, decreased appetite, constipation, dizziness, sweating, upset urination, sexual problems, decreased libido, urinary retention, weight changes, slower growth in children, increased heart rate and blood pressure, aggressiveness, mania, hypomania.
Atomoxetine was initially studied as a drug for the treatment of depression, but did not show a favorable risk / benefit ratio in clinical trials. Subsequently, the manufacturing company Eli Lilly and Company examined the atomomxetine for the treatment of ADHD. Many patients observe a pronounced antidepressant effect of atomoxetine (when used with other antidepressants).
The efficacy of atomoxetine in the treatment of psychogenic overeating was investigated. The average dose of atomoxetine was 106 mg / day. The study showed that "the use of atomoxetine was associated with a significantly higher rate of reduction in episodes of overeating, weight loss and BMI." The authors of the study concluded that atomoxetine can be effective in the short-term treatment of psychogenic overeating.

 Cart
Cart