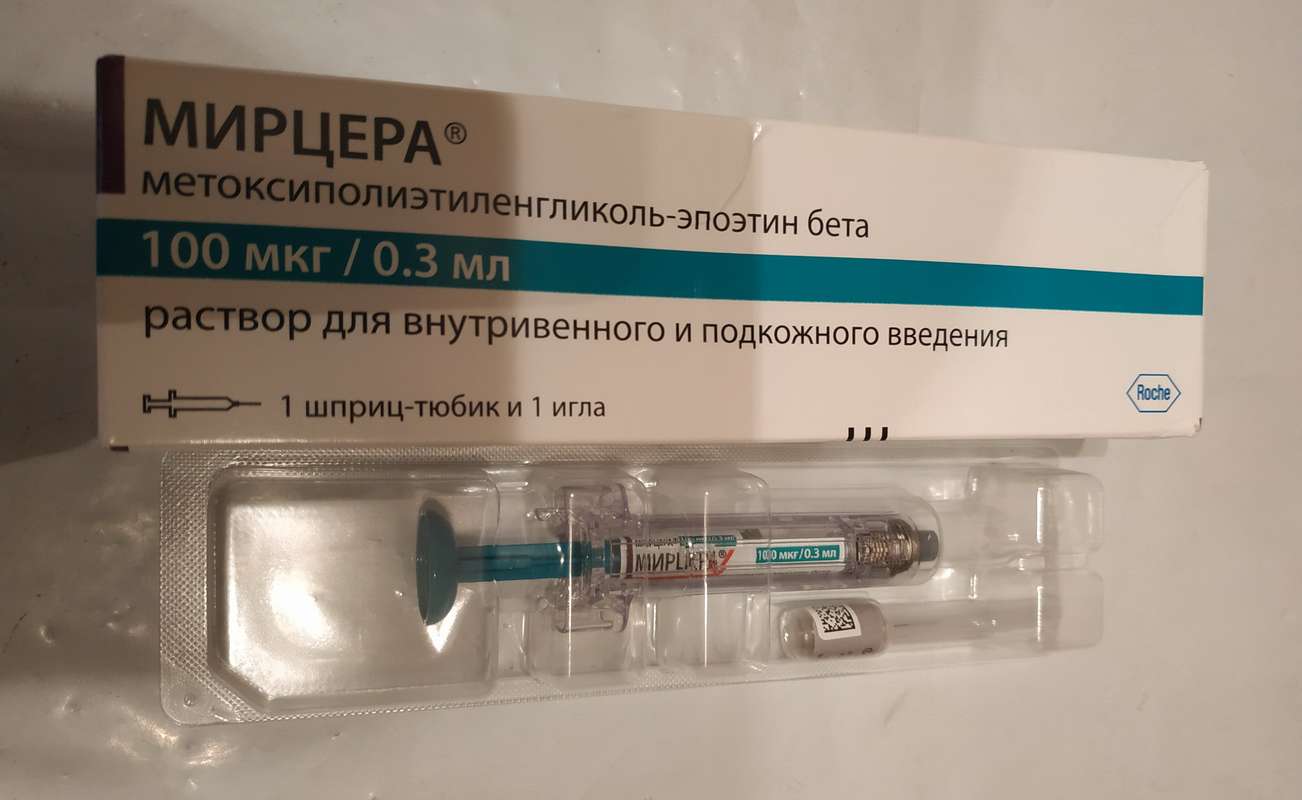
Myrcera is a chemically synthesized representative of a new class of long-acting erythropoietin receptor activators. Methoxypolyethylene Glycol-epoetin beta is a covalent conjugant of a recombinant DNA-derived protein and linear methoxypolyethylene glycol (PEG).
Mircera has a different activity at the receptor level from erythropoietin and is characterized by a longer association with the receptor and faster dissociation from the receptor, reduced specific activity in vitro and increased activity in vivo, as well as an increased half-life, which allows you to inject Mircera 1 time per month.
Indications
Anemia in chronic renal failure (according to the NKF K/DOQI classification - in chronic kidney disease).
Method of administration and dosage
Mircera can be administered either subcutaneously (subcutaneously) or intravenously (intravenously).
The drug should be administered n / a in the shoulder area, the anterior surface of the thigh or the anterior abdominal wall.
The Hb content should be monitored once every two weeks before its stabilization and periodically after stabilization.
Correction of the dose of the drug is carried out no more than 1 time per month.

 Cart
Cart