Bromdihydrochlorphenylbenzodiazepine - Active Substances. Instruction and Application, Dosage
28 Dec 2016
Name: Bromdihydrochlorphenylbenzodiazepine
The Latin name of the substance Bromdihydrochlorphenylbenzodiazepine
Bromdihydrochlorphenylbenzodiazepinum (genus Bromdihydrochlorphenylbenzodiazepini)
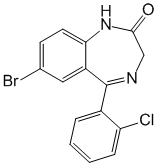
Chemical name: 7-Bromo-5- (2-chlorophenyl) -1,3-dihydro-2H-1,4-benzodiazepin-2-one
Formula - C15H10BrClN2O
Therapeutic substances Bromdihydrochlorphenylbenzodiazepine- anxiolytics
The nosological classification (ICD-10)
F00 Dementia in Alzheimer's disease (G30 +)
F03 Unspecified Dementia
F05 Delirium, not induced by alcohol or other psychoactive substances
F07.2 Post-contusion syndrome
F10.3 abstinence
F11 Mental and behavioral disorders due to use of opioids
F20 Schizophrenia
F23 Acute and transient psychotic disorders
F25 Schizoaffective disorders
F30 Manic episode
F32 Depressive episode
F40.0 Agoraphobia
F40.8 Other phobic anxiety disorders
F41.0 Panic disorder [episodic paroxysmal anxiety]
F41.1 Generalized anxiety disorder
F42 Obsessive-compulsive disorder
F43.0 Acute stress reaction
F43.1 Post-traumatic stress disorder
F43.2 Disorder adaptive reactions
F44 Dissociative [conversion] disorders
F45 Somatoform disorders
F45.2 Hypochondriacal disorder
F48 Other neurotic disorders
F51.0 Insomnia inorganic etiology
F60 Specific personality disorders
F60.2 antisocial personality disorder
F60.3 Emotionally unstable personality disorder
F63 Disorders of habit and impulse
F79 Mental retardation, unspecified
F95 Teaks
G40 Epilepsy
G41 Status epilepticus
G47.0 Disorders of falling asleep and maintaining sleep [insomnia]
R25.2 Cramp and spasm
R45.0 Nervousness
R45.4 Irritability and anger
Z100 * CLASS XXII Surgical practice
CAS code - 1753-57-2
Characteristics substance Bromdihydrochlorphenylbenzodiazepine
Anxiolytic.
White or light white crystalline powder. Practically insoluble in water and ether, slightly soluble in ethanol, it is difficult in chloroform.
Pharmacology of Bromdihydrochlorphenylbenzodiazepine
Mode of action - anxiolytic, anticonvulsant, muscle relaxant, central, hypnotic, sedative.
Stimulates the benzodiazepine receptors, it increases the sensitivity of GABA receptors to mediator, increases the braking effect of GABA in the CNS. Reduces the excitability of the subcortical brain structures inhibits polysynaptic spinal reflexes. It reduces emotional stress, reduces anxiety, fear, anxiety. It suppresses the spread of excitation arising in the epileptogenic foci in the cortex, the thalamus, and limbic structures of the brain by increasing presynaptic inhibition (increased focus activity is not reduced).
Well absorbed from the gastrointestinal tract. Time to reach C max -. 1-2 hours is metabolized in the liver. T1 / 2 -. 6-10 hours excreted by the kidneys. Reduced liver and kidney function slows down the clearance and leads to accumulation.
Application of the Bromdihydrochlorphenylbenzodiazepine substance
Neurotic, neurosis, psychopathic and psychopathic state, accompanied by anxiety, fear, increased irritability, tension, and emotional lability; sleep disorders, obsessive-compulsive disorder, panic disorder, phobias, reactive psychosis; vegetative lability, hypochondriacally-senestopatic syndrome; seizures of various etiologies, temporal and myoclonic epilepsy, status epilepticus, muscle rigidity, teak, athetosis, hyperkinesis; premedication (as a component of induction of anesthesia); in the complex therapy of opioid and for abuse syndrome, schizophrenia (febrile form, increased sensitivity to antipsychotics); prophylaxis of conditions of fear and emotional stress.
Contraindications of Bromdihydrochlorphenylbenzodiazepine
Severe myasthenia gravis, severe liver damage (cirrhosis, infectious disease) and kidneys; poisoning other tranquilizers, antipsychotics, hypnotics, narcotics, alcohol.; pregnancy, breast-feeding.
Pregnancy and breast-feeding
Contraindicated during pregnancy. At the time of treatment should stop breastfeeding.
Side effects of Bromdihydrochlorphenylbenzodiazepine substance
From the nervous system and sensory organs: dizziness, headache, drowsiness, muscle weakness, impaired memory, concentration, coordination, paradoxical excitement, ataxia, mydriasis.
From the digestive tract: dry mouth, nausea, diarrhea.
Allergic reactions: skin rash, pruritus.
Other: dysuria, dysmenorrhea, loss of libido, addiction, drug dependency.
Interaction of Bromdihydrochlorphenylbenzodiazepine
(Mutually) effect of neuroleptics, tranquilizers, sleeping pills, narcotic, analgesic, anticonvulsant drugs, and alcohol.
Overdose of Bromdihydrochlorphenylbenzodiazepine
Symptoms: marked depression of consciousness, cardiac and respiratory activity.
Treatment: control of vital functions, maintaining respiratory and cardiovascular activity, symptomatic therapy. Specific antagonist flumazenil (w / 0.2 mg - optionally up to 1 mg - 5% glucose solution or 0.9% sodium chloride solution).
Dosing and Administration of Bromdihydrochlorphenylbenzodiazepine
Inside, in / m or / in (bolus or infusion). The mode set is strictly individual, depending on the indications of the disease, tolerance and others.
For quick relief of fear, anxiety, psychomotor agitation, as well as autonomic epileptic and psychotic states: in / m or / in, the initial dose for adults - 0.5-1 mg (0.5-1 ml of 0.1% solution ), the average daily - 3-5 mg, in severe cases, up to 7-9 mg. Premedication: / slow 4.3 mg (3.4 ml of a 0.1% solution).
When administered a single dose for adults is usually 0.5-1 mg (with sleep disorders - 0.25-0.5 mg), the average daily - 1.5-5 mg 2-3 hours (typically 0.5 -1 mg in the morning and during the day and 2.5 mg at night), the maximum daily - 10 mg.
Neurotic, neurosis, psychopathic and psychopathic states: Inside, the initial dose of 0.5-1 mg 2-3 times a day, 2-3 days (if necessary) - up to 4-6 mg / day; in severe agitation initial dose - 3 mg / day, then rapidly increasing the dose until a therapeutic effect.
Neurological Practice (disease with increased muscle tone), inside of 2-3 mg 1-2 times a day or in / m, 0.5 mg 1-2 times a day.
Status epilepticus, serial seizures: in / m or / in, starting with a dose of 0.5 mg. In the treatment of epilepsy - 2.10 mg / day.
Alcohol withdrawal: inside, 2.5-5 mg / day.
The course of treatment with oral - 2 weeks (if necessary - up to 2 months), at parenteral administration - up to 3-4 weeks. To use caution in the elderly and in children (a daily dose reduced by a factor of 2-3).
Precautions substance Bromdihydrochlorphenylbenzodiazepine
Note that the combination of anxiety with depression may attempt suicide.
To apply caution in conjunction with other psychotropic drugs. Should not be administered concurrently with MAO inhibitors and phenothiazines.
With prolonged use (over several months), particularly at high doses, may addiction, drug dependence. Abolition of chlordiazepoxide should be done gradually by reducing the dose to reduce the risk of withdrawal syndrome characterized by tremor, cramps, abdominal and muscle cramps, vomiting, sweating. In abrupt cancellation also possible agitation, anxiety, autonomic disorders, insomnia.
Do not take chlordiazepoxide more than 4 weeks without re-evaluation of the patient to decide whether to continue therapy.
The probability of side effects is higher in the elderly.
Prolonged use should be periodically monitored picture peripheral blood and liver function.
There are reports that in the treatment of chlordiazepoxide mental patients and children with the aggressive behavior traits observed paradoxical reactions (psychomotor agitation, rage, etc.).. In the case of paradoxical reactions, treatment should be discontinued immediately.
It should be borne in mind that chlordiazepoxide can reduce mental alertness in children.
It must be borne in mind that there are reports of effects on blood coagulation in patients taking oral anticoagulants simultaneously and chlordiazepoxide (in a clinical study of the causes and consequences of this interaction has not been established). There are some reports of exacerbation of porphyria under the action of chlordiazepoxide. Hypoproteinemia may predispose to increase the frequency of sedative side effects.
During treatment and for 3 days after its cancellation should be deleted reception alcohol; drivers of vehicles and people whose work requires quick mental and physical reactions, and is also associated with high concentration of attention, should not engage in professional activities during this period.
Special instructions for Bromdihydrochlorphenylbenzodiazepine
As an equalizer avoids or reduces some of the side effects that can be applied mesocarb. Be wary of the organic cerebral insufficiency. The lifting should be done gradually by reducing the dose to reduce the risk of withdrawal symptoms.
Should not be used during the drivers of vehicles and people skills relate to the high concentration of attention. At the time of treatment should abandon admission alcohol.
Trading names of drugs with Bromdihydrochlorphenylbenzodiazepine working substance
Trade Name Index
Bromdihydrochlorphenylbenzodiazepine,Trankvezipam, Phezanef, Phezipam, Phenazepam tablets, phenazepam, Phenzitat, Phenorelaxan, Elzepam

 Cart
Cart