Doctor Doping Shop
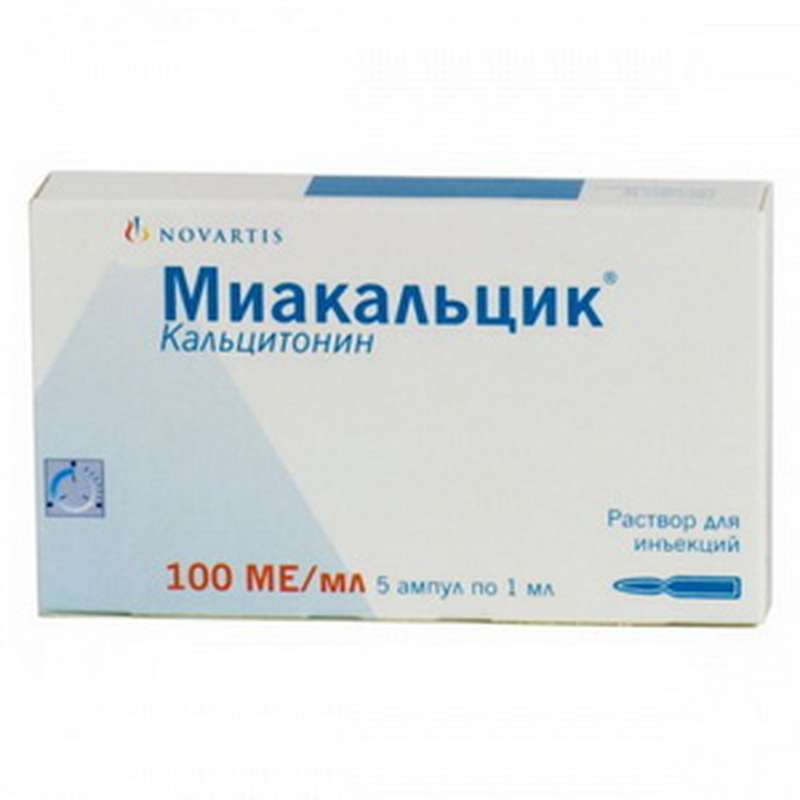
Miacalcic injection 100ME 1ml per vials, 5 vials
USD 108.00
In stock
Be the first to review this item
Myacalcic - hypocalcemic, inhibiting bone resorption.
The hormone produced by C-cells of the thyroid gland is an antagonist of parathyroid hormone and together with it participates in the regulation of calcium metabolism in the body.
The structure of all calcitonins is represented by one chain of 32 amino acids and a ring of 7 amino acid residues at the N-terminal, the sequence of which is not the same for different species. Since salmon calcitonin has a higher affinity for receptors (compared to mammalian calcitonins), its effect is expressed to the greatest extent both in strength and in duration.
By suppressing the activity of osteoclasts due to action on specific receptors, salmon calcitonin significantly reduces the rate of bone tissue exchange to normal levels in conditions with an increased rate of resorption, for example, in osteoporosis. Both in animals and in humans it has been shown that Miakaltsik has analgesic activity in bone pain, which is apparently due to direct exposure to nCTC.
Already after a single application of Myacalcic in the form of injectable solution or nasal spray, a clinically significant biological response is observed in man, which is manifested by increased urinary excretion of calcium, phosphorus and sodium (due to a decrease in tubular reabsorption) and decreased hydroxyproline excretion.
Prolonged parenteral or intranasal administration of Miacalcic leads to a significant reduction in the level of biochemical markers of bone metabolism, such as pyridinoline, serum C-telopeptides (sCTX) and bone isoenzymes of AP.
Calcitonin in parenteral administration reduces gastric and exocrine pancreatic secretion. These properties of the drug Myacalcic determine its effectiveness in the treatment of acute pancreatitis.
The use of Myacalcic nasal spray results in a statistically significant (1-2% increase) in bone mineral density in the lumbar vertebrae, which is already determined in the first year of treatment and lasts up to 5 years. The drug Miakaltsik ensures the maintenance of mineral density in the femur.
The use of Miacalcic nasal spray at a dose of 200 IU / day leads to a statistically and clinically significant decrease (by 36%) of the risk of developing new vertebral fractures in the group of patients who received Myacalcic (in combination with vitamin D and calcium preparations), compared to with a group of patients receiving a placebo (in combination with the same drugs). In addition, in the group of patients treated with Myacalcic (in combination with vitamin D and calcium preparations), a 35% decrease in the incidence of multiple vertebral fractures was noted compared to the placebo group (in combination with the same drugs). Calcitonin reduces gastric and exocrine pancreatic secretion.
Pharmacokinetics
Bioavailability of salmon calcitonin, both with intramuscular and subcutaneous injection, is about 70%, and with intranasal application 3-5% in relation to the bioavailability of the drug used parenterally. Cmax of the drug in plasma is achieved within 1 h, and prip / k introduction - for about 23 min. Myacalcic is rapidly absorbed through the nasal mucosa and its Cmax in plasma is reached during the first hour (on average, about 10 min).
The apparent VSS is 0.15-0.3 l / kg. Binding to plasma proteins - 30-40%. Up to 95% of calcitonin and its metabolites are excreted in the urine, and only 2% - in unchanged form. The T1 / 2 drug is about 1 hour with the / m introduction; 1-1.5 h - with subcutaneous injection and about 20 min - with intranasal.
With repeated prescriptions of the drug intranasal cumulation was not noted. When the drug was used in doses exceeding the recommended values, its concentrations in the blood were higher (as confirmed by an increase in AUC values), but the relative bioavailability did not increase.
Determining the concentration of salmon calcitonin in plasma, as well as the concentrations of other polypeptide hormones, is of little value, because the therapeutic effectiveness of the drug can not be predicted from the concentration level. Thus, the activity of Myacalcic should be evaluated by clinical efficacy.
Salmon calcitonin does not penetrate the human placental barrier.
Indications:
- ·bone pain associated with osteolysis and / or osteopenia;
- Paget's disease (deforming osteitis);
- neurodystrophic diseases (synonyms - algoneurodystrophy, Zudeck's atrophy), caused by various etiological and predisposing factors, such as posttraumatic painful osteoporosis, reflex degeneration, humerus syndrome, causalgia, drug neurotrophic disorders;
- postmenopausal osteoporosis (both early and late stages).
- primary osteoporosis - senile osteoporosis in women and men;
- secondary osteoporosis, in particular caused by glucocorticoid therapy or immobilization.
- Hypercalcemia and hypercalcemic crisis, caused by the following factors;
- osteolysis caused by malignant tumors (carcinoma of the breast, lungs, kidneys, myeloma, etc.);
- hyperparathyroidism;
- immobilization;
- intoxication with vitamin D;
- relief of emergency conditions and long-term treatment of chronic hypercalcemia - until the effect of specific therapy of the underlying disease appears;
- acute pancreatitis (as part of combination therapy).
Contraindications:
Hypersensitivity to synthetic calcitonin salmon and other components of the drug.
Special instructions:
A doctor or nurse should instruct the patients in detail who independently make themselves hypodermic injections of the drug.
Before using Myacalcic, you should visually check the condition of the ampoule and solution. The ampoule of the preparation should not be damaged, the solution should be clear, colorless and without foreign inclusions. After a single use of Myacalcic, the unused solution of the drug remaining in the ampoule should be disposed of. Before subcutaneous or intramuscular injection, the solution of Myacalcic should be heated to room temperature. With prolonged use of the drug Myacalcic in patients can form antibodies to calcitonin; However, this phenomenon usually does not affect clinical efficacy. The phenomenon of "slippage", observed mainly in patients with Paget's disease, receiving the preparation Myacalcic for a long time, is probably due to saturation of binding sites, rather than the formation of antibodies. After a break in treatment, the therapeutic effect of Myacalcic is restored. In Paget's disease, as well as in other chronic diseases with an increased level of bone turnover, the duration of treatment with Myacalcic should be several months to several years. Against the background of treatment, the concentration of alkaline phosphor in the blood and the excretion of hydroxyproline in the urine are reduced, and often normalized. However, it should be borne in mind that in some cases, after the initial decline, the values of these indicators may again rise. In these cases, when deciding whether to cancel the treatment or the time of its resumption, the doctor should be guided by the clinical picture. One or several months after discontinuation of treatment, bone metabolism disorders may occur again, in which case a new treatment with Miacalcic® will be required.
Since salmon calcitonin is a peptide, there is a possibility of systemic allergic reactions. There have been reports of allergic reactions, including isolated cases of anaphylactic shock, which occurred in patients receiving Mycalcic. If susceptibility to a patient's sensitivity to salmon calcitonin is suspected, skin tests should be performed prior to treatment, using a diluted sterile solution of Miacalcic.
The drug Miakaltsik, solution for injection, practically does not contain sodium (less than 23 mg).
Influence on ability to drive vehicles and work with mechanisms. The effect of Myacalcic on the ability to drive vehicles and work with mechanisms has not been studied. Some of the side effects of the drug, such as dizziness and visual disturbances, can adversely affect the ability to drive and perform potentially dangerous activities requiring increased concentration and speed of psychomotor reactions.
Note. With prolonged therapy, the formation of antibodies to calcitonin is possible, but this is not generally affected by clinical efficacy. The phenomenon of addiction, which is observed mainly in patients with Paget's disease receiving long-term therapy, may be a consequence of the saturation of binding sites and obviously has nothing to do with the formation of antibodies. The therapeutic effect of Myacalcic is restored after a break in treatment.
Suggested Use:
Subcutaneously, intramuscularly, intravenously.
Osteoporosis. The drug is given SC or IM in a daily dose of 50 or 100 IU daily or every other day (depending on the severity of the disease). In order to prevent progressive loss of bone mass at the same time with the use of the drug Myacalcic recommended the appointment of adequate doses of calcium and vitamin D.
Bone pain associated with osteolysis and / or osteopenia. The daily dose is 100-200 IU daily. The drug is administered IV, dropwise (in physiological saline) or SCI or IM in several administrations - until a satisfactory clinical effect is achieved. The dose should be adjusted taking into account the patient's response to treatment.
To achieve a complete analgesic effect, it may take several days. In long-term therapy, the initial daily dose is usually reduced and / or the interval between administrations is increased.
Paget's disease. P / to or in / m in a daily dose of 100 IU daily or every other day. The duration of treatment is at least 3 months; if necessary, it can be more. The dose should be adjusted taking into account the patient's response to treatment.
Hyperkalciemia. Emergency treatment of hypercalcemic crisis. Since IV infusion is the most effective mode of administration, it should be given preference for treatment of urgent and other severe conditions.
Myacalcic is injected intravenously into the drip for a minimum of 6 hours, at a daily dose of 5-10 IU / kg in 500 ml of saline. It is also possible in / in a jet slow injection, in which the daily dose should be divided into 2-4 injections during the day.
Long-term treatment for chronic hypercalcemia. Daily sc or to / m in a daily dose of 5-10 IU / kg once or in 2 injections. The dosage regimen should be adjusted taking into account the dynamics of the patient's clinical state and biochemical parameters. If the volume of the required dose of Myacalcic is more than 2 ml, then I / m injections should be preferred, which should be carried out in different places.
Neurodystrophic diseases. It is extremely important to make an early diagnosis. Treatment should be started immediately after confirmation of the diagnosis. P / to or in / m in a daily dose of 100 IU for 2-4 weeks. It is possible to continue treatment with the introduction of 100 IU every other day for up to 6 weeks, depending on the dynamics of the patient's condition.
Acute pancreatitis. Myacalcic is used as a part of combined conservative treatment. Enter IV in a drip in a dose of 300 IU (in saline) for 24 hours for 6 consecutive days.
Packaging:
- Comes in original packaging. Item is brand new and unopened.
Storage:
- Keep away from direct sunlight.
- Keep locked and away from children.
- Store in dry place at room temperature.
- Do not exceed storage temperature higher than 25 C
Important notice- the outer box design may vary before prior notice!
Related products


 Cart
Cart