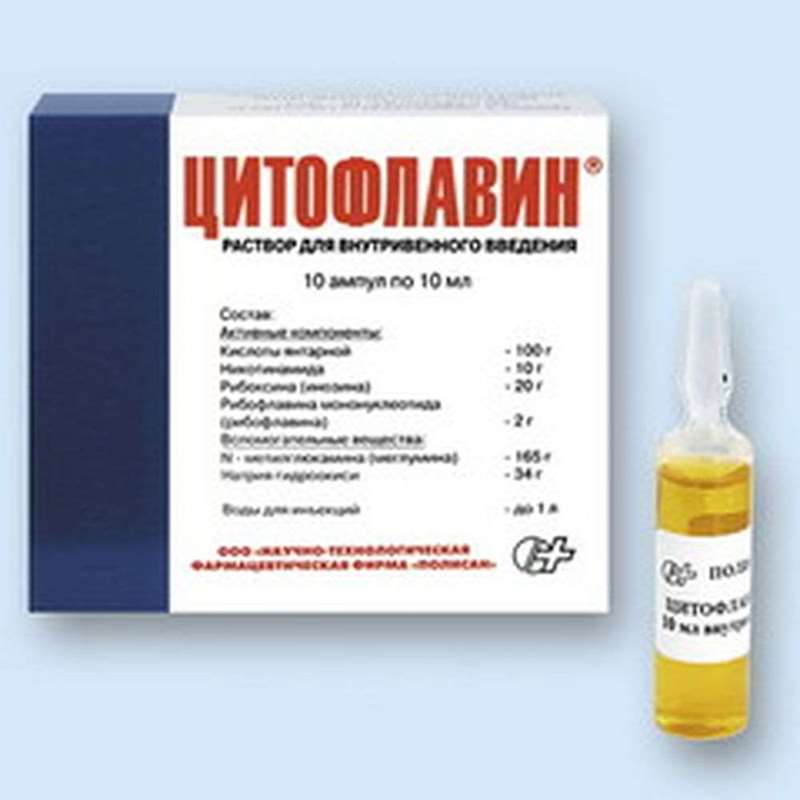
Pharmacological effects are caused by the combined action of a part of the drug Cytoflavin components.
Cytoflavin (Inosinum + Nicotinamidum + Riboflavinum + Acidum succinicum, Inosine + Nicotinamide + Riboflavin + Acid Succinic) - activation promotes aerobic metabolism of cells, resulting in increased levels of glucose utilization contributes to an increased level of fatty acid beta-oxidation and resynthesis γ-aminobutyric acid in neurons.
Cytoflavin increases the stability of the membranes of nerve and glial cells to the effects of ischemia, resulting in a decrease in the concentration of proteins neurospecifical characterizing the level of degradation of the main structural components of the nervous tissue.
Cytoflavin improves coronary and cerebral blood flow, activates metabolic processes in the central nervous system, restores impaired consciousness, promotes regression of neurological symptoms and improving cognitive functions of the brain. It has a rapid awakening effect in postanesthetic oppression of consciousness.
In applying the drug Cytoflavin in the first 12 hours after the onset of stroke observed beneficial for ischemic and necrotic processes in the affected area (reduction of the hearth), recovery of neurological status and reduction of disability in the long term.
Pharmacokinetics:
Citoflavin has high bioavailability.
With intravenous infusion at a rate of about 2 ml / min (calculated on undiluted Citoflavin) succinic acid and inosine are utilized almost instantly and are not detected in the blood plasma.
Succinic acid - peak concentration is determined within the first minute after administration to a further rapid decrease in cumulative and without returning it to the level of background values due to metabolization of water and carbon dioxide.
Inosine is metabolized in the liver to form inosine monophosphate, followed by its oxidation to uric acid. In small quantities excreted by the kidneys.
Nicotinamide is rapidly distributed in all tissues, crosses the placenta and breast milk, is metabolized in the liver with the formation of N-methylnicotinamide, excreted by the kidneys. plasma half-life period of about 1.3 hours, the equilibrium distribution of volume - about 60 liters, total clearance - about 0.6 L / min.
Riboflavin is unevenly distributed: the highest number in the myocardium, liver, kidney. plasma half-life period is about 2 hours, the equilibrium distribution volume - 40 liters, total clearance - about 0.3 L / min. It penetrates through the placenta and breast milk. Communication with plasma proteins - 60%. Excreted by the kidneys, partly in the form of metabolites; in high doses - preferably in an unmodified form.
Testimony:
In adults in the treatment of:
- acute stroke.
- The effects of cerebrovascular disease (the effects of cerebral infarction, cerebral atherosclerosis).
- toxic and hypoxic encephalopathy in acute and chronic poisoning, endotoxemia, postanesthetic oppression of consciousness, as well as for the prevention and treatment of hypoxic encephalopathy during cardiac surgery with cardiopulmonary bypass.
In children (including preterm gestation 28-36 weeks) in combination therapy in the neonatal period:
- cerebral ischemia.
Contraindications:
Hypersensitivity, patients who are on mechanical ventilation, while reducing the partial pressure of oxygen in arterial blood is less than 60 mm Hg. Art., the period of breastfeeding.
Be wary - nephrolithiasis, gout, hyperuricemia.
Side effects:
Headache, pain or discomfort in the epigastric region. Allergic reactions such as skin redness and itching. Adverse reactions include: transient hypoglycemia, hyperuricemia, gout exacerbation of concomitant.
If any of these instructions side effects are compounded or you notice any other side effects not mentioned in the instructions, inform your doctor.
Special instructions:
Introduction newborn drug (premature) children to carry out under the control of acid-base balance of capillary blood at least 2 times a day (both before and during therapy). If possible, control the rate of serum lactate and glucose.
The rate of administration containing Citoflavin solution should be reduced or temporarily discontinue infusion in neonates (premature) children:
- Are receiving mechanical ventilation, when the signs of the mixed (respiratory-metabolic) alkalosis, threatened by the development of cerebral circulatory disorders;
- When a stored spontaneous breathing and respiratory support CPAP method or from receiving a mixture of air and oxygen through a mask when a laboratory signs of metabolic alkalosis, or threatened by the appearance of frequent episodes of apnea.
Patients treated diabetes be under the control of blood glucose index.
Perhaps the intense staining of urine yellow.
Citoflavin does not affect the ability to drive.
Suggested Use:
In adults:
Cytoflavin used only intravenously at a dilution of 100-200 ml of 5-10% dextrose or 0.9% sodium chloride solution.
1. In acute stroke drug is administered as early as possible from the beginning of the disease in the volume of 10 ml for administration at intervals of 8-12 hours for 10 days. In severe form of the disease a single dose of 20 ml.
2. If the consequences of cerebrovascular diseases (consequences of cerebral infarction, cerebral arteriosclerosis) the drug is administered in a volume of 10 ml per administration once daily for 10 days.
3. hypoxic encephalopathy and toxic drug is administered in a volume of 10 ml for administration twice a day every 8-12 hours for 5 days. In a coma in a volume of 20 ml for administration at a dilution of dextrose per 200 ml of solution. When postanesthetic depressed once in the same doses. In the treatment of hypoxic encephalopathy with cardiac surgery with cardiopulmonary bypass is administered 20 ml of the drug dilution in 200 ml of 5% dextrose solution for 3 days prior to surgery, on the day of surgery and 3 days after surgery.
In children (including premature) in the neonatal period with cerebral ischemia Cytoflavin daily dose of drug was 2 ml / kg / day. The calculated daily dose administered intravenously (slowly) after dilution in 5% or 10% dextrose solution (in a ratio of at least 1: 5). Time first introduction - the first 12 hours after birth; the optimal time to begin therapy are the first 2 hours of life. Recommended prepared solution administered by an infusion pump at a rate of 1 to 4 ml / hr, ensuring uniform delivery of the drug into the blood stream throughout the day, depending on the calculated volume daily solutions basic therapy, the patient's hemodynamic status indicators and acid-base status. The course of treatment an average of 5 days.
Drug interactions:
Succinic acid, nicotinamide and inosine are compatible with other drugs. Riboflavin reduces the activity of certain antibiotics (tetracycline, erythromycin, lincomycin), is incompatible with streptomycin. Ethanol, tricyclic antidepressants, tubular secretion blockers reduce the absorption of riboflavin, and thyroid hormones speed up his metabolism.
Packaging:
- Comes in original packaging. Item is brand new and unopened.
Storage:
- Keep away from direct sunlight.
- Keep locked and away from children.
- Store in dry place at room temperature.
- Do not exceed storage temperature higher than 25 C
Important notice- the outer box design may vary before prior notice!

 Cart
Cart