17 Apr 2018
Hormones are the most famous regulators of processes in the body. Their functions and chemical structure have been studied quite well, which makes it possible to create medications on their basis and successfully carry out, for example, substitution therapy. On the second place in the illumination are located neurotransmitters. These compounds preferentially link the nerve cells to each other, thereby activating or inhibiting various regions and processes. Influence on neurotransmitters is somewhat more complicated, but many drugs have been developed that affect metabolism, ejection, reverse capture, etc., hence the neurotransmitter system is adjustable.
At this stage in the development of medicine and pharmacology, the neuropeptides are least studied and controlled. Nevertheless, they take an active part in almost all processes in the body, regulate the most important behavioral and adaptive reactions, control circadian rhythms, food and sexual behavior, affect hormones and neurotransmitters. It is impossible to ignore this topic, as well as to collect all the data on it. According to the results of the studies, the data does not always coincide, sometimes they are directly opposite, which makes the description of neuropeptides difficult, due to the lack of a single classification, there is still much confusion. Thus, information on neuropeptides is not systematized enough, nevertheless this material is the most complete in Russian and includes a significant number of compounds not listed in other reviews.
Neuropeptides (NP) - biologically active compounds that participate in the regulation of metabolism, maintenance of homeostasis, affect immune processes, play an important role in the mechanisms of memory, learning, sleep, etc. The NP is characterized by a relatively short sequence of a chain consisting of amino acid residues, as a rule, it is 5-52 elements.
NPs are formed as a result of sequential hydrolytic decomposition of the precursor peptide, for example, several substances can form from the same initial molecule, which at each stage may have biological activity different from the initial and final products. The NP precursors are synthesized in the body of the cell by translating the gene encoding the peptide, then the proteases point split the long molecule into shorter chains that can later undergo transformation or remain unchanged. If it is a neuron, then the NP is transported to the presynaptic terminal, from which it is released into the synaptic cleft. Some neuropeptides are able to perform the functions of mediators, carrying out a direct transmission of the nerve impulse, others change the metabolism of the cell, acting as a neuromodulator. NP can transmit the signal not only to close distances, the receptors to some of them are at a sufficient distance from the site of synthesis, which makes it possible to compare them with hormones. The functions of many NPs duplicate each other, nevertheless everyone has a unique spectrum of activity. This feature allows you to more accurately transmit the signal and regulate the interaction of different parts of the body, coordinate a complex system of continuously occurring processes that somehow affect each other and require constant corrections. The vast majority of neuropeptides affect the "slow" metabotropic receptors associated with G-proteins. In addition, NP can change the activity of each other and some hormones (more often inhibit or activate their synthesis), which leads to the launch of cascade reactions.
Many neuropeptides are synthesized to a greater or lesser degree in various organs and tissues, and only some of them are strictly specific for certain zones. Distribution of NP in the body is not uniform, there are places that are most characteristic for each substance, but in small amounts they are determined in virtually all tissues. In the neural tissue, NPs are present in unmyelinated C-type fibers and small A-delta-type myelinated fibers. In the spinal cord, NPs are synthesized by the cells of the dorsal horns of the ganglia, then transported along the axons to the nerve endings, where they can act as neurotransmitters. In synaptic terminals, NPs are able to act together with non-protein neurotransmitters. A neuropeptide may be colocalized with one or more mediators, which leads to some enhancement or modification of the action. If their selection coincides in time, then the effect depends on both of them, but they can also be separated separately, which leads to the realization of the biological effect of each of them separately from each other.
At present, there is no complete and comprehensive classification of NPs. Attempts were made to develop it on the basis of the chemical structure, functions or place of synthesis. However, many NPs are able to perform several functions depending on the location, similar in structure of the compound - responsible for different processes, and various substances of origin being agonists. NP found in any tissues were not always specific to them and were later found in other organs. In addition, at the moment, new compounds are opening, which can not be attributed to any of the existing groups, since their functions are not fully understood. The classification of NPs by families can be regarded as the most complete and functional, since it takes into account the greatest number of features of these substances.
Classification of Neuropeptides:
- Hypothalamic liberins and statins
- Opioid peptides
- Melanocortins
- Vasopressin-tocine
- Pancreatic peptides
- Glucagon secretions
- Cholecystokinin
- Tachykinins
- Motilin
- Neurotensins
- Bombesins
- Kininy
- Angiotensins
- Peptides encoded by a gene similar to the calcitonin gene
- Atriopeptides
- Endozepines
- Galanin
- Endothelins
Hypothalamic liberins and statins
Tyroliberin, corticoliberin, lyuliberin, somatoliberin, somatostatin, melanostatin. The first family includes neuropeptides synthesized by the hypothalamus. They were combined into one group according to the topological feature and had mainly the function of stimulation (liberins) or inhibition (statins) of the synthesis of pituitary hormones. According to the chemical structure, the hypothalamic neuropeptides differ considerably from each other and have different precursors. In addition to influencing the structures remote from the site of synthesis, the NPs of this group can influence neighboring neurons, depressing or vice versa stimulating each other's formation. As they studied, representatives of the first family were found in other organs and tissues and found the ability to influence the emotional state, food and sexual behavior, the regulation of the sleep-wake cycle, the provision and launch of stress-protective mechanisms, the stimulation of immune processes, neurogenesis and many others.
Opioid peptides
The family of opioid peptides is characteristic of the majority of representatives of the amino acid sequence Tyr-Gly-Gly-Phe. Accordingly, OP works on opioid receptors, mainly μ (MOP), δ (DOP), and κ (KOP), associated with G-proteins. Due to the ability to bind to the listed receptors, OPs have a naloxone-inhibiting, morphine-like analgesic and sedative effect. EP have a variety of biological effects. With regard to the impact on behavior, they have the ability to influence aggression, motivation for satisfaction, sexual attraction, food saturation, stressor adaptive processes, drug dependence, sedation, modulation of pain sensitivity, etc. In addition, they participate in neurodegenerative processes, damage to brain tissue due to trauma and ischemia.
Opioid peptides (OP) are widely distributed in the central and peripheral nervous systems, GIT, serum, are produced not only by neurons, but also by cells of the endocrine and immune systems. Most opioid peptides are formed from common protein progenitors - proopiomelanocortin (α-MSH, γ-MSH, β-MSH, ACTH, β-endorphin, α-endorphin, γ-endorphin, β-lipoprotein (β-LPH), γ-LPH , CLIP), prodinorphine (dinorphin A, dinorphin B, α-neoendorfin, β-neoendorfin, dinorphin-32, leimorfin), preproenkephalin (leu-enkephalin, meth-enkephalin, amidorfin, adrenorphine, peptide B, peptide E, peptide F), prepro- nociceptin, preproorpharin (nociceptin (orfanin FQ)), prepro-NPFF (NP FF, NP AF, NP SF), and others.
Enkephalins are short peptide chains, from 5 amino acid residues. Typical members of the family are leu-enkephalin and met-enkephalin, named for the fifth amino acid, respectively leucine and methionine. In addition, the group includes DTLET and DAMGO. They act mainly on the δ-opioid receptors. Both neuropeptides have a pronounced morphine-like analgesic, sedative effect. They take part in the formation of behavioral reactions. Their participation in many neurodegenerative pathologies is proved.
Endorphins α and β are products of hydrolysis of POMC and contain 16 and 31 amino acid residues, respectively. They participate in the motivation of alcoholic behavior, nociceptive reactions, stress response, regulation of circadian rhythms. Β-endorphin is less specific to receptors and can activate to a greater or lesser extent all three of the number of opioids.
Dinorphins are formed during the conversion of prodinorphine and contain in their structure a sequence of leu-enkephalin. The most important action is the central and peripheral nociceptive process. The group includes dinorfin A and dinorfin B (rimorphin), containing respectively 17 and 13 amino acid residues. In addition, α-, β-neoendorfin is formed. All of them activate mainly κ-opioid receptors. Their selectivity is due to the presence of arginine and lysine at the C-terminus. If the precursor proteolysis does not take place completely, a so-called "big" dinorphine is formed, which includes dinorphine A and B, and has the same properties, but is more selective for BCD.
Dermorphin and deltorphin-specific agonists of the μ- and δ-opioid receptors, respectively, consisting of 7 amino acid residues. They participate in reducing the threshold of epileptic readiness, have a pronounced analgesic effect, stimulate the release of β-endorphin. They differ in the presence of the D-amino acid in the second position, which makes them more resistant to enzymatic hydrolysis.
Hemorphins are products of proteolytic decomposition of hemoglobin, have an affinity for μ-opioid receptors. Participate in the analgesic reaction and the development of euphoria after exercise.
Endomorphin-1 and -2 are tetrapeptides, show the greatest specificity for the μ-opioid receptors from the family. Have a pronounced and long analgesic effect.
Nocystatin contains 17 amino acid residues in its composition. Reduces pain sensitivity. Studies are under way to create on its basis analgesic agents that are not addictive and morphine-like addiction.
Β-Casamorphin consists of 7 amino acid residues and is formed by the hydrolysis of casein. It is able to activate μ-opioid receptors, stimulate the immune system and increase food intake.
Leumorfin (leimorfin) is formed from the precursor of preprodinorphin. Have sufficient affinity for opioid receptors and for biological action is comparable with other OP.
Adrenorphine is synthesized mainly in the adrenal glands. Has the ability to influence nociceptive processes, as well as other peptides of the family.
A group of peptides with anti-opioid activity enhance the pain response, increase anxiety, stimulate the release of ACTH and corticosterone, inhibit morphine-induced effects. Prevent the formation of dependence on alcohol and morphine, affect the development of withdrawal syndrome in animals with morphine dependence.
Presented are:
Neuropeptides AF and SF consist of 18 and 11 amino acid residues, respectively. The neuropeptide FA consists of 8 amino acid residues. Receptors to it are located mainly in the spinal and supraspinal zones containing a large number of endogenous opioids.
Nociceptin (Orphanin FQ) - consists of 17 amino acid residues and has a structure similar to that of opioid peptides. The receptors for nociceptin are similar to opioid receptors, linked to adenylate cyclase. When these receptors are activated, potassium channels are activated and calcium is inhibited. Nociceptin and its receptors are most widely represented in the cortex, olfactory nuclei, amygdala, hippocampal formation and dorsal horns of the spinal cord. Takes part in the processes of memory, learning, stressful reactions. In experimental models showed the ability to reduce anxiety. Activation of the nociceptin receptors leads to analgesia, but it interferes with the action of opioids.
Melanocortins
Adrenocorticotropin (ACTH) is a hormone synthesized in the anterior pituitary gland, the main function of which is stimulation of adrenal corticosteroid production. It is proved that he is able to synthesize and other parts of the brain, and in addition to the hormonal function to act as a neurotransmitter, to participate in the regulation of higher cortical functions such as memory, attention, training.
A-β-γ-melanotropins (melanocyte-stimulating hormones) are formed from proopiomelanocortin. Synthesis occurs most intensively in the median lobe of the pituitary gland. The receptors for MSH are associated with G-proteins and are divided into 2 types: MCHR1 and MCHR2. The expression of Type 1 receptors is highest in the cortex, hippocampus, amygdala and nucleus accumbent, suggesting that these neuropeptides are involved in the development of pathologies such as mood disorders and schizophrenia. This is confirmed by the introduction of antagonists to this type of receptor, which caused anxiolytic and antidepressant effects. A-MSH stimulates the formation of pigment in the skin, participates in mental processes - memory and learning, sleep, aggression, modulates inflammation in the brain, blocks the synthesis of gliosis of tumor necrosis factor. Γ-MSG has a lesser effect on the pigment metabolism, but enhances the steroidogenic function of ACTH. All MSG are able to participate in the regulation of gastrointestinal function, immune processes, cell growth and mitosis, and eating behavior.
Vasopressin-tocine
Vasopressin and oxytocin are produced in the hypothalamus, along the axons enter the posterior lobe of the pituitary gland, from which they are released into the blood. They have a strong effect on the formation of behavioral reactions, such as affection, sexual, parental behavior. Under stress, they participate in the creation of protective mechanisms. In addition, they can influence blood pressure, reduce smooth muscle, metabolism.
Mesotocin, isotocin, vasotocin is composed of 10 amino acid residues, having a common initial sequence of 6 amino acids. Synthesize mainly in the posterior lobe of the pituitary gland together with oxytocin and vasopressin and are similar to them for biological effects, but less active.
Predecessors - preprovazopressin-neurofizin II (vasopressin, neurofizin II), preproxitocin-neurofizin I (oxytocin, neurofizin I).
Pancreatic peptides
The neuropeptide Y consists of 36 amino acid residues. Distributed in the brain (hypothalamic and cortical regions, hippocampus, thalamus) and peripheral nervous system, postganglionic sympathetic fibers, adrenal glands, megakaryocytes and platelets. There is evidence of a change in the distribution of NP in the neuronal population of the prefrontal cortex in ontogenesis in pathological disorders. Oppressing the selection of the transmitter from the nerve endings. The effect is manifested by hypotension, hypothermia and respiratory depression, secretion of water and electrolytes in the intestine, regulation of circadian rhythms and motivational behavior. Is able to participate in the regulation of eating behavior, so, with its chronic introduction into the central nervous system, the body weight increased. During the period of food intake decrease, the amount of neuropeptide Y in arched and paraventricular nuclei increased. With the breakdown of the neuropeptide Y, the resulting substances can act as its agonists and antagonists, which is determined by their chemical structure.
The tyrosine tyrosine peptide (PYY) has a similar structure to the neuropeptide Y and differs from it with the additional amino acid residue -Tyr. By function, they are just as similar and competing for binding to the same receptors.
Pancreatic polypeptide (PPY) is synthesized by PP-cells of the islets of Langerhans pancreas. It has 36 amino acid residues. By the mechanism of action it is a cholecystokinin antagonist, suppresses the secretory activity of pancreatic cells and stimulates the production of gastric juice, delaying food in the stomach.
The precursors are prepro-NPY (NPY), prepro-PPY (PPY), prepro-PYY (PYY).
Glucagon secretions
Glitsentinya is an intermediate product of the formation of glucagon from preproglucagon. Until the end of its properties are not studied, it is assumed that the spectrum of its activity is similar to that of glucagon.
Glucagon (GRP) contains 27 amino acid residues. The largest amounts are contained in the gastrointestinal tract and brain, secreted by some tumors, where it stimulates mitosis, has a trophic effect on normal and neoplastic cells, including autocrine stimulation of small cell lung cancer proliferation. Participates in the regulation of glucose metabolism, with an increase in its level stimulates the synthesis of insulin and inhibits eating behavior. In the central nervous system improves the processes of memory formation, takes part in the body's response to stress. It takes care of the regulation of sleep-wake cycles, body temperature, appetite and satiety. Modulates the activity of macrophages. Regulates the secretion of pancreatic and hydrochloric acid enzymes in the stomach, stimulates the contraction of smooth muscles and the release of certain intestinal hormones, including gastrin.
VIP (vasoactive intestinal peptide) consists of 28 amino acid residues. Widely represented in the central nervous system, especially in the cerebral cortex, where it participates in the formation of behavioral reactions, has a positive effect on the processes of learning and memory, sexual behavior. Can act as a mediator in serotonergic and cholinergic systems. On the periphery causes the expansion of the bronchi, vessels (including cerebral).
Secretin contains 27 amino acid residues. It is mainly produced by brain structures, adrenals and intestines. Most strongly, its effect is manifested in the cells of the gastrointestinal tract, where it leads to relaxation of smooth muscles and increased secretion of hormones by the pancreas.
Gastrin is mainly produced by G-cells of the stomach and pancreas. A "large" gastrin is formed, which consists of 34 amino acid residues, gastrin-17 and gastrin 14, which contain respectively 17 and 14 amino acids. On the functions performed they are similar, since all contain the same active center. Increase the secretion of hydrochloric acid, pepsin, bicarbonates, secretin, cholecystokinin, somatostatin and some other peptides involved in digestion. Stops the emptying of the stomach. It leads to the expansion of the stomach vessels by increasing the production of prostaglandin E. Participates in the regulation of eating behavior, reducing the motivation for food search.
Predecessors - preprogastrin (gastrin), preproglycagon (glycinthin, glucagon), preprosecretin (secretin), prepro-VIP (VIP).
Cholecystokinin
Cholecystokinin contains 33 amino acid residues. Influencing the structure of the central nervous system affects the emotional state, leads to the activation of behavior aimed at obtaining food, has an antidepressant effect. It is of great importance in the regulation of the functions of the gastrointestinal tract - it stimulates secretion in the pancreas, motility of the gallbladder and intestines. In the decay of cholecystokinin, products that have their own action are formed. Some of them can reduce the effects of morphine and enkephalins with respect to pain sensitivity. Preecessor is preprocholecystocinin.
Tachykinins
This group includes NPs that have a β-preprotachykinin precursor and contain the-Gly-Leu-Met sequence at the C-terminus. Biological effects are mediated by exposure to receptors associated with G-protein. Tahikinins can also act as neurotransmitters and are widely represented in various tissues of the body. The main physiological effects are the regulation of the tone of the smooth muscles of the intestine, bronchi, participate in behavioral reactions, nociceptive processes, inflammatory processes.
The substance contains in its composition 11 amino acid residues. It was opened in 1931. and is the most studied of the family. It is synthesized mainly in the central nervous system - the amygdala, the septum, the hippocampus, the hypothalamus and the gray matter in the brain water pipeline, which are involved in the formation of anxiety and depression. Occurs in the posterior horns of the spinal cord is a neuromodulator in primary afferent fibers and unmyelinated C-type fibers. It has a wide spectrum of physiological effects - it can influence the blood pressure level, capillary permeability, smooth muscle contraction, possesses secretory action, participates in the control of secretion of prolactin and digestive hormones. The synthesis of substance P is enhanced by dopamine: thus, it was revealed that when dopanergic fibers are damaged, mRNA expression is reduced, which corresponds not only to the formation of substance P, but also to enkephalins and dinorphine. Participates in the transmission of a pain signal. The ability of substance P to influence training, sleep, resistance to stress is studied.
Neurokinins (A, B and K) are similar in their effects to substance P, but have a different specificity for the receptors. They change the excitability of nerve cells, have the ability to exert an anti-inflammatory effect - their effect leads to vasodilation and increase in their permeability, the release of prostaglandin E2, cytokines and amines by mast cells and leukocytes. In addition to these physiological effects, they participate in the transmission of a nerve impulse.
Cassinin consists of 12 amino acid residues. Takes part in lowering blood pressure, stimulating contraction of smooth muscles. There is evidence that cassinin has anticonvulsant activity.
Motilin
In its composition contains 22 amino acid residues. It is produced mainly in the gastrointestinal tract, where it affects mainly the motor function - it strengthens the tone of the lower sphincter-esophagus, stimulates the emptying of the stomach and the motility of the large intestine. Stimulates the production of insulin and somatostatin by the pancreas. In the central nervous system, the highest concentration is found in the hippocampus. The effect of motilin on mental functions has not been studied enough, it is assumed that it affects food behavior.
The predecessor is prepromotilin.
Neurotensins
Neurotensin is found mainly in the hypothalamus, mesocorticolimbic and nigrostriatic zones, ventral cover, septum, cingulate gyrus, small intestinal mucosa. The peptide has a strong hypotensive effect, leads to a reduction in smooth muscle, reduces body temperature, increases the glucose and glucagon content, can bind to mast cell receptors. Neurotensin has in some sense a hormonal action - in the pituitary gland enhances the secretion of LH and FSH. There are data on its ability to influence sexual behavior, the development of stress reactions and nociceptive processes. It is established that dysfunction in the neurotensin system occurs in mental illnesses, in particular schizophrenia. It is assumed that the effect on its metabolism can have an effect in psychotic states, without causing at the same time an increase in weight and cataleptic manifestations. However, clinically significant evidence for this was not obtained. Associated with dopaminergic, serotonergic, GABAergic, glutamatergic and cholinergic systems.
Neuromedins N and U (NMN, NMU) are secreted in the cells of the central nervous system and gastrointestinal tract. Receptors to them are located in neurons, cells of the small intestine, pancreas, stomach, lymphocytes, monocytes, muscle fibers of the uterus. Exposure to NMU leads to a decrease in body weight by reducing appetite. In the regulation of immune processes increases inflammation, activating the mast cells. NMN can act as a neurotransmitter.
Xenopsy is involved in the regulation of smooth muscle tone.
The precursor is propreneurotensin.
Bombesins
Bombesin consists of 14 amino acid residues. It is a powerful activator of the hypothalamic-pituitary-adrenal axis, participates in the regulation of the body's stress reaction, affects the memorization processes. In addition, it regulates the consumption of ethanol. Physiological effects consist in vasoconstriction, decrease in body temperature, regulation of secretory processes in the gastrointestinal tract, takes part in autocrine stimulation of cell proliferation and growth of small lung cancer cells.
Gastrin-releasing peptide (GRP) consists of 27 amino acid residues. It is common in the brain tissue, intestines, lungs, immune system, and others. Its main functions can be called regulation of sleep-wake cycles, thermoregulation, effects on appetite and satiety, modulation of macrophage activity, increased secretion of enzymes by the pancreas, hydrochloric acid in the stomach , a reduction in smooth muscles, the release of gastrin in the intestine, and participation in the regulation of respiration at the level of the brainstem. GRP stimulates the mitotic activity of cells, including small cell lung cancer.
Litorin has many properties of the family, in particular, the ability to regulate body temperature.
Kinines
Kinins have a wide spectrum of activity and are a link in the regulation of vascular tone, blood coagulation and fibrinolysis. They are synthesized in most tissues, including the CNS.
Bradykinin consists of 9 amino acid residues. Its effect leads to relaxation of the smooth muscles of the walls of the vessels, bronchi, uterus, intestine. It takes part in the regulation of hemostasis, electrolyte balance, permeability of capillaries, local inflammatory reactions and pain sensitivity. The muscle fibers outside the vessels act in the opposite way, leading to their constriction, which is important in the development of inflammation and pain intensification.
Kallidin consists of 10 amino acid residues and differs from bradykinin by the presence of a lysine residue at the beginning of the chain. By physiological effects similar to bradykinin.
The predecessor is the kininogen.
Angiotensins
Angiotensins I, II, III are synthesized in the central nervous system and other tissues and organs. The most studied functions of these peptides are the regulation of the state of the cardiovascular system, water-salt metabolism, and blood pressure. Angiotensins are products of sequential hydrolytic cleavage from the terminal portion of several amino acids. Thus, angiotensin II is formed from angiotensin II and further with a shortening of the chain. The most powerful of the group is angiotensin II, which is formed under the influence of renin and PDA. It plays a role in the formation of mechanisms of arterial hypertension. It is part of the renin-angiotensin-aldosterone system. It is associated with the adrenergic system and tachykinins by the mechanism of action. It has been proven that angiotensins participate in learning processes, memory formation, motivation, internal reinforcement, pain sensitivity and control of emotions.
The precursor is the preprotein angiotensinogen.
Peptides encoded by a gene similar to the calcitonin gene
Calcitonin (contrinsular hormone) consists of 32 amino acid residues. It is produced mainly by C-cells of the thyroid gland. Calcitonin-like immunoreactivity is found in the pituitary gland, cerebrospinal fluid, lungs, thymus, intestine, liver, bladder. In the brain, the highest content of calcitonin is found in the zone surrounding the posterior part of the hypothalamus, mid elevation and pituitary gland. He takes an active part in the regulation of the water-salt balance. Reduces the content of calcium and phosphorus in the blood plasma, which leads to a change in the metabolism and activity of cell membranes. Has analgesic and anorectic effect, leads to vasodilation, hypotension, hyperglycemia, stimulates gluconeogenesis and glycogenolysis.
Calcitonin-gene-related peptide (CGRP) consists of 37 amino acid residues. In fairly large amounts, it occurs in the central and peripheral nervous, cardiovascular, genitourinary, GIT and C-cells of the thyroid gland. Has the ability to influence blood pressure, depending on the attendant factors leads to hypo- or hypertension, is a strong vasodilator, causes tachycardia, is involved in maintaining the tone of the coronary vessels and modulating the pain sensitivity, affects food behavior and cerebral circulation.
The precursor is prepro-CALC (calcitonin).
Endozepines
The peptide inhibitor of diazepam binding (DBI) consists of a large number of amino acids. Biological activity has both DBI itself and its fragments - endozepin-6 and octadecaneuropeptide (6 and 18 amino acid residues in its composition, respectively). These peptides are in large numbers in the central nervous system and are ligands of benzodiazepine receptors. By structure - strong antagonists GABA, which determines their biological effects. The level of endozapines increases in brain tissue under stress and aging. It has been proved that these peptides play a role in the formation of reactions to stress and the development of anxiety states - when intragastric administration has an anxiogenic and proconflicogenic effect, the search for their antagonists can lead to the creation of a new type of anti-anxiety drugs.
The predecessor is BDI in isoforms 1, 2, 3.
Galanin
Galanin consists of 29 amino acid residues. Receptors to it are located in the hippocampus, hypothalamus, amygdala, preoptic zone, supraoptic, arcuate nucleus. Receptors are divided into 3 types and they are all associated with G-protein. In experiments it was shown that when exposed to galanin receptors, anxiolytic and antidepressant effects can be obtained. When combined with other substances, it can exert the most diverse influence - when colocalized with acetylcholine takes part in mnestic processes, the role in the development of Alzheimer's disease, with noradrenaline - the formation of social status, with vasopressin and oxytocin in supraoptic and paraventricular nuclei acquires the ability to influence osmoregulation. Formed in the hypothalamus stimulates the secretion of LH and takes part in the regulation of the feedback of the HGH axis. It is able to inhibit the secretion of glutamate and the electrical activity of the arcuate nucleus.
The predecessor is pre-progalanin.
Endothelins
Endothelin I, II, III are synthesized predominantly in the vascular endothelium, their expression is present in the neural tissue. They are powerful vasoconstrictors. Together with other peptides and hormones play an important role in the regulation of the endothelium, the development of renal ischemia, hypertension, bronchial asthma, heart failure and cerebral vascular pathologies. The main active peptide of the group is endothelin I. Endothelins have different precursors expressed by different genes.
In addition to the neuropeptides represented in this classification, there are a large number of compounds not included in it. To date, several hundred NPs have been opened, which for various reasons can not be attributed to existing groups. Here are some of them.
Orexins
Orexins belong to the group of hypothalamic peptides and include orexin A and orexin B, consisting of 33 and 28 amino acid residues, respectively. Both peptides interact with the OXR1 and OXR2 receptors. Neurons secreting orexins are contained in the perifornical zone of the lateral hypothalamic field. Despite the fact that there are not many of them, these neurons strongly branch, having connections with various parts of the brain, such as paraventricular, dorsomedial arcuate nucleus of the hypothalamus, blue spot, posterior hypothalamic field, spinal cord. Orexins control food intake, participate in the regulation of circadian rhythms and the development of stress reactions, sexual behavior. Their concentration in the hypothalamus increases with fasting, but intracerebral administration causes only a brief increase in appetite without a noticeable effect on the total amount of food consumed. Narcolepsy and catalepsy are associated with the absence of orexin, as they can inhibit both phases of sleep and prolong the period of wakefulness. Orexins increase the activity of the sympathetic nervous system and maintain muscle tone, which is important with increasing physical activity and maintaining it at a high level. In addition, they can positively influence the secretion of HGH-axis hormones. It is assumed that orexins can play a role in the development of diseases such as Huntington's chorea, Parkinson's disease, obstructive sleep apnea syndrome, type II diabetes.
Leptin
Leptin consists of 35 amino acid residues. The predecessor is preproleptin. It is synthesized mainly in adipocytes and only in small amounts in other organs and tissues, including such parts of the brain as the cortex, hippocampus, cerebellum, basal ganglia, trunk. The greatest number of receptors for leptin in the brain is concentrated in the ventrobasal and ventromedial hypothalamus. With the accumulation of white adipose tissue in the body, the content of leptin increases and, accordingly, when fasting decreases. The peptide plays an important role in food behavior and energy metabolism - it reduces the need for food, suppresses appetite and speeds up the metabolic processes at the periphery. Directly or indirectly regulates the synthesis of hormones that affect food behavior, reducing the synthesis of oryxigenic and increasing anorexigenic. There is evidence that leptin may act as a neuroprotective agent, a neuroplastic factor. Leptin inhibits the development of excitotoxicity of glutamate and limits the damage caused by oxidative stress. In the hippocampus, under the influence of the hippocampus, the formation of free radicals is suppressed. In dopaminergic neurons it is able to stabilize mitochondrial membranes and to limit the phenomena of oxidative stress due to expression of uncoupling proteins of UCP2. Limits apoptosis in cells. Has an anticonvulsant effect. Affects memory processes, contributing to the consolidation of short-term and long-term. It is suggested that a reduction in leptin levels may be positively associated with the development of Alzheimer's disease, because it limits amyloidogenesis, by inhibiting β-secretase and activating α-secretase, and also participates in τ-phosphorylation processes, reducing the formation of abnormal τ-protein, facilitating elimination β-amyloid. It is suggested that it may be important in the development of Huntington's chorea and Parkinson's disease. In addition, it regulates blood pressure and vascular tone.
DSIP (delta-sleep-inducing peptide)
DSIP (delta-sleep-inducing peptide) consists of 9 amino acid residues. Until now, the gene, its coding, predecessor and specific receptors with the genes encoding them has not been identified. DSIP is found in the neurons of Brock's diagonal ligament, the ventral septum, the anterior hypothalamus, in the areas with gonadotropin-releasing hormone-reactivity, melanin concentrating hormone, thyroid-stimulating hormone, peptides of the secretory cells of the gastrointestinal tract, melanocorticotropes of the intermediate lobe of the pituitary gland, brainstem, pituitary and epiphysis; has specific differences in localization. Often, DSIP is colocalized with catecholamines in the chromafin granules of the adrenal medulla. With intraperitoneal administration of its rats, an increase in the content of norepinephrine and serotonin in the cerebral cortex and an increase in adrenaline without a change in the concentration of dopamine have been established, due to which it can exert a inhibitory effect on the central nervous system. There are data on stimulation of GABA production and prophylaxis. DSIP blocks the stimulating effect of glutamate by reducing the sensitivity of the "fast" ionotropic NMDA receptors of the glutamatergic system of the brain and the subsequent reduction of glutamate stress-induced excitotoxicity. Has a membrane-stabilizing effect on neuronal, erythrocyte and leukocyte membranes. Increases the activity of c-Fos gene expression, which is a marker of neuronal activity, of various parts of the limbic system, playing a trigger role in the development of emotional reactions to stress and forming their neurotransmitter integration, which leads to activation of somatovegetative manifestations. It has an anti-stress action and helps to create a state of "pre-adaptation".
The involvement of DSIP in the regulation of sleep-wake cycles is controversial, different authors provide various data, from the ability to suppress to stimulation of sleep phases. Has antiepileptic and anticonvulsant activity. The peptide can participate in analgesic processes by enhancing the binding of met-enkephalin to the OP. Enhances the release of melatonin by cleaving the remainder of tryptophan from the terminal portion of the molecule. It affects the decrease in MAO activity, inhibition of corticoliberin synthesis, stimulation of the synthesis of somatotropin, somatostatin and lylyberyrin. Determines naloxone-dependent analgesia. It is able to reduce the level of lipids and cholesterol, the cholesterol coefficient of atherogenicity. DSIP is able to prevent oxidative modification of proteins during physiological aging. Has hyperglycemic activity, which may be due to an increase in the concentration of catecholamines, which reduce the release of insulin against the background of increased production of glucagon.
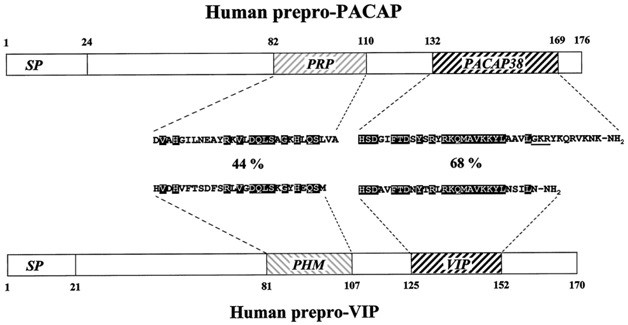
PACAP (a peptide that activates pituitary adenylate cyclase)
PACAP (a peptide that activates pituitary adenylate cyclase) contains 38 amino acid residues. The structure and functions are similar to VIP. PACAP is widely distributed in brain structures and peripheral organs, including the endocrine system. The greatest number of neurons containing a peptide is found in the hypothalamus, in particular in the supraoptic and paraventricular nuclei synthesizing vasopressin and oxytocin. The peptide stimulates their production by activation of cAMP. Can act in the hypothalamus as a neurotransmitter and neuromodulator in the regulation of hormone secretion. Modulates vasopressin and oxytocin in the regulation of blood pressure and osmosis of cells, takes part in modulating the function of the cerebellum with physical activity. It is suggested that PACAP is involved in regulating the rhythm of melatonin production in the pituitary and, accordingly, affects circadian rhythms. Participates in the regulation of food behavior, having an anorexigenic effect by activating cAMP in supraoptic and paraventricular nuclei. It is important in ontogenesis, inhibiting the programmed apoptosis in the developing brain, stimulating the growth of neurites, decreasing the number of mitotically dividing cells and promoting the differentiation of neuroblasts, has a neuroprotective effect in neurotoxicity caused by an increased concentration of glutamate. Consequently, in the developing brain, PACAP acts as a neurotrophic factor, and in the developed brain as a neuroprotective agent. By activating the pituitary adenylate cyclase, the peptide stimulates the release of hormones.
BINP (neurotrophic peptide from the damaged brain)
BINP consists of 13 amino acid residues. It protects brain cells from excitotoxicity of glutamate, promotes survival of cholinergic neurons of the septum and dopaminergic neurons of mesencephalon in the primary culture of the neonatal brain.
It is difficult to overestimate the role of neuropeptides. Their study over time could open prospects for the creation of drugs and artificial regulators of the body's processes. Now a lot of research is being done and attempts are being made to create preparations based on them, but the matter is complicated by the insufficient study of all the properties of neuropeptides and their interaction with other systems. As an example of such studies, the following table can be cited.
Examples of the results of II and III phases of clinical trials of ligandine neuropeptide receptors in mental disorders.


 Cart
Cart