Buspirone - Active Substances. Instruction and Application, Dosage
26 Dec 2016
Name: Buspirone
The Latin name of the substance Buspirone
Buspironum (genus. Buspironi)
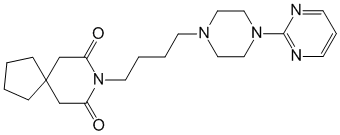
chemical name 8- [4- [4- (2-pyrimidinyl) -1-piperazinyl] butyl] -8-azaspiro [4.5] decane-7,9-dione (and hydrochloride)
Formula - C21H31N5O2
Therapeutic agents of Buspirone: Dopamine-mimetics, anxiolytics
The nosological classification (ICD-10)
F10.3 abstinence
F40.0 Agoraphobia
F41.0 Panic disorder [episodic paroxysmal anxiety]
F41.1 Generalized anxiety disorder
F42 Obsessive-compulsive disorder
F45 Somatoform disorders
CAS code - 36505-84-7
Characteristics substance Buspirone
Non-benzodiazepine anxiolytic structure. Buspirone hydrochloride - a white crystalline powder. It is soluble in water. The molecular weight of 422.0.
Pharmacology
Mode of action - anxiolytic.
It has high affinity for transformation (agonist) and postsynaptic (partial agonist) serotonin receptor subtype 5-HT1A. Decreases the synthesis and release of serotonin, the activity of serotonergic neurons, including in the dorsal raphe nucleus. Selectively blocking (antagonist) pre- and postsynaptic dopamine D2-receptors (having moderate affinity) and increases the rate of excitation of the midbrain dopamine neurons. Some data indicate the presence of the impact on other neurotransmitter systems. No affinity for the benzodiazepine receptors have no effect on the binding of GABA.
The effect is gradually manifested after 7-14 days, and reaches a maximum after 4 weeks. Do not have any negative effect on psychomotor functions, it does not cause tolerance, dependence and withdrawal symptoms. It does not potentiate the effect of alcohol. Studies have shown the effectiveness of buspirone in autism, obsessive-compulsive disorders, premenstrual syndrome, sexual dysfunction, and alleviation of symptoms during smoking cessation.
When long-term administration to rats (within 2 years) at doses of 133 exceeds MRDC or mice (for 1.5 s) at doses 167 times higher than the MRDC, carcinogenic effects were found. Not mutagenic. In rats and rabbits at doses 30 times higher than MRDC and influence on fertility, and adverse effects on the fetus was observed. Buspirone and its metabolites enter the breast milk of rats.
Once inside quickly and completely absorbed from the gastrointestinal tract and undergoes extensive first-pass metabolism. At the "first pass" through the liver and dealkylated hydroxylated (bioavailability - 4%). N-dealkylated metabolite - 1 pirimidinilpiperazin is pharmacologically active (anxiolytic activity is 1/4 that of buspirone). Upon receiving the 20 mg dose Cmax achieved after 40-90 minutes and is 6.1 ng / ml. Binding to plasma proteins - 95%. Simultaneous eating reduces the rate of absorption, but increases the amount of unchanged drug that has reached the systemic circulation (AUC and Cmax of buspirone increased by 84 and 116%, respectively) due to inhibition of first-pass clearance). Excreted by the kidneys (29-63%) in the form of metabolites and unaltered (1%), and gastrointestinal tract (18-38%). T1 / 2 of buspirone - 2-3 h, T1 / 2 of the active metabolite - 4,8 hours.
Application of the substance Buspirone
Generalized anxiety disorder, panic disorder, vegetative dystonia syndrome, alcohol withdrawal syndrome (adjuvant therapy), depression (adjuvant therapy).
Contraindications Buspirone
Hypersensitivity, severe violations of the kidneys and liver, glaucoma, myasthenia gravis, pregnancy, breast-feeding.
Restrictions to application Buspirone
Age 18 years (Safety and efficacy have not been determined).
Pregnancy and breast-feeding
When pregnancy is used only when necessary.
Category effects on the fetus by FDA - B.
At the time of treatment should stop breastfeeding.
Side effects of substance Buspirone
From the nervous system and sensory organs: dizziness (12%), somnolence (10%), headache (6%), nervousness (5%), fatigue (4%), insomnia (3%), decreased ability to concentrate attention (2%); extrapyramidal disorders (very rare); ≤2% - blurred vision, confusion or depression, weakness, numbness; ≤1% - neurological symptoms (muscle weakness, tingling, pain or weakness in the hands or feet, uncontrolled movements of the body).
Cardio-vascular system and blood (blood, hemostasis): ≤1% - tachycardia / palpitations.
From the digestive tract: nausea (8%), dry mouth (3%), diarrhea (2%); ≤1% - vomiting, constipation; loss of appetite.
Other: ≤1% - myalgia, cramps, spasms, or muscle stiffness, rash, sweating.
Interaction of substance Buspirone
In combination with MAO inhibitors may develop a hypertensive crisis.
Overdose of substance BuspironeSymptoms: nausea, vomiting and other gastrointestinal disorders, dizziness, cramps, drowsiness.
Treatment: gastric lavage, the appointment of activated charcoal, symptomatic therapy. Dialysis is ineffective.
Dosing and Administration of Buspirone Substances
Inside. The recommended initial dose - 5 mg 3 times a day, if necessary, it can be increased by 5 mg every 2-3 days. The average daily dose - 20-30 mg. The maximum single dose - 30 mg daily - 60 mg.
Precautions for Buspirone substance
With caution used in conjunction with antipsychotics, antidepressants, cardiac glycosides, antihypertensive and antidiabetic agents, oral contraceptives. In renal and liver failure mild to moderate liver cirrhosis designate smaller doses and under strict medical supervision. During the period of treatment should be deleted alcohol. At the beginning of treatment with use caution during the drivers of vehicles and people skills relate to the high concentration of attention.
special instructions
Anxiety or tension associated with everyday stress usually does not require treatment with an anxiolytic.
Trading names of drugs with working substance BuspironeTrade Name
Spitomin

 Cart
Cart